COVID-19 conversation and collaboration
Aetion is committed to advancing COVID-19 research through our work with industry, including FDA, biopharma, and working groups such as the COVID-19 Evidence Accelerator. This page features a snapshot of our and our collaborators’ latest thinking on how real-world data can inform understanding of and response to coronavirus disease.
Multi-stakeholder workshop on global COVID-19 regulatory agilities
Aetion’s Lowell Schiller, Chief Legal and Regulatory Officer, participated in a workshop sponsored by Avalere Health and Merck to discuss innovative methods and tools adopted by regulators during the COVID-19 pandemic, and how they should be used going forward. These included tools and regulatory agilities to accelerate evidence generation, including advances in the use of real-world evidence, to accelerate the development of vaccines and therapeutics without compromising quality, safety, or efficacy. Many of these approaches, including advances in RWE, will benefit patients, developers, and the public health in many contexts outside the pandemic setting.
We are honored that Aetion’s work with FDA was featured as a case study in this workshop, showcasing Aetion’s role in advancing the use of RWE during the COVID-19 pandemic.

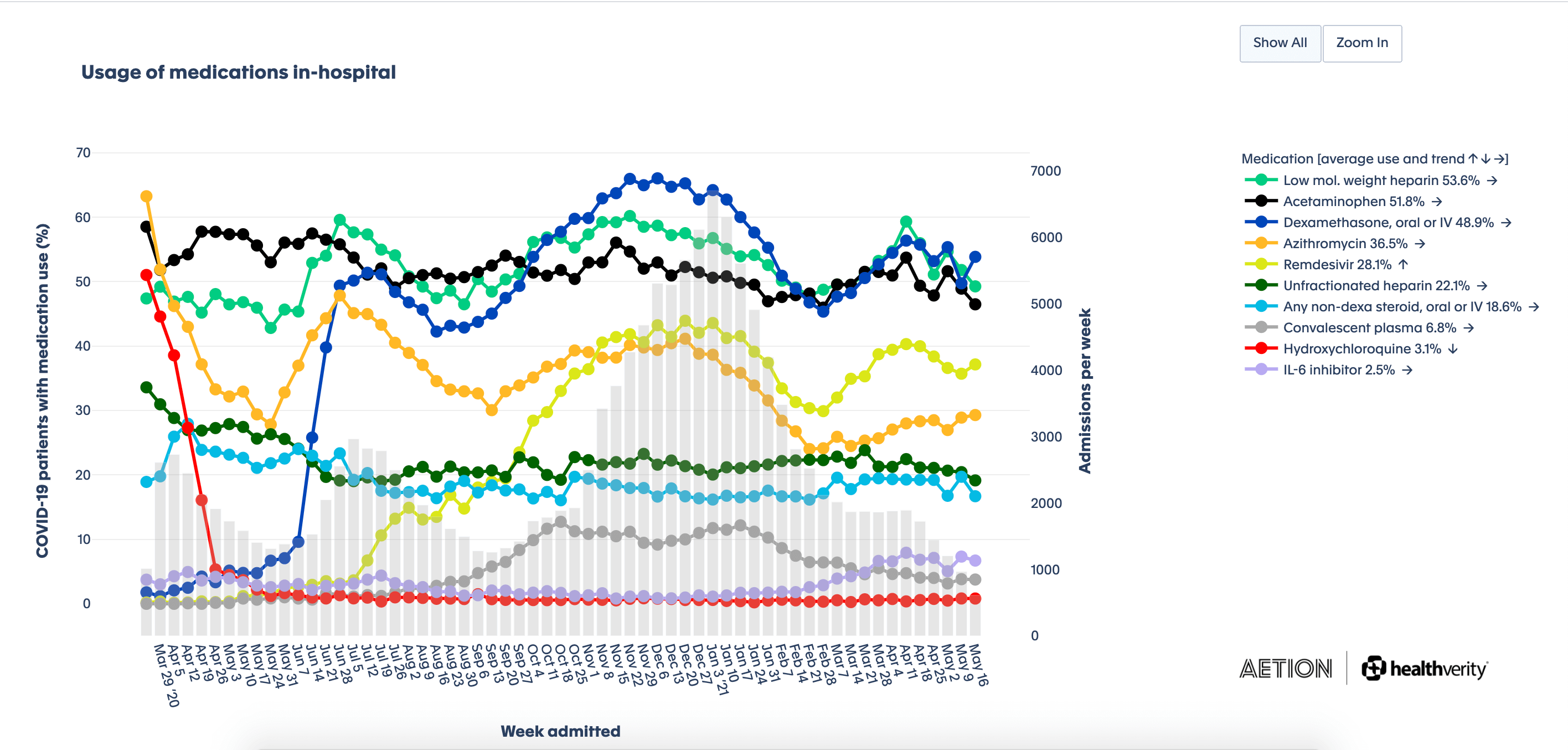
Usage of medications in-hospital
This chart—generated in Real-Time Insights and Evidence, a solution powered by Aetion and HealthVerity—shows U.S. hospital visits over time, with corresponding use of common medicines used to treat COVID-19.