Discover
Visualize rapid, validated insights through real-world data.
Datavant Completes Acquisition of Aetion. Learn more →
Turning data into decisions that shape the future of healthcare
Aetion, now a Datavant company, builds software for—and deep partnerships with—the world’s life sciences developers, regulators, and purchasers. Our work is to know, show, and share how medical innovations work in the real world.
Driving scalable evidence generation with trusted technology and real-world data
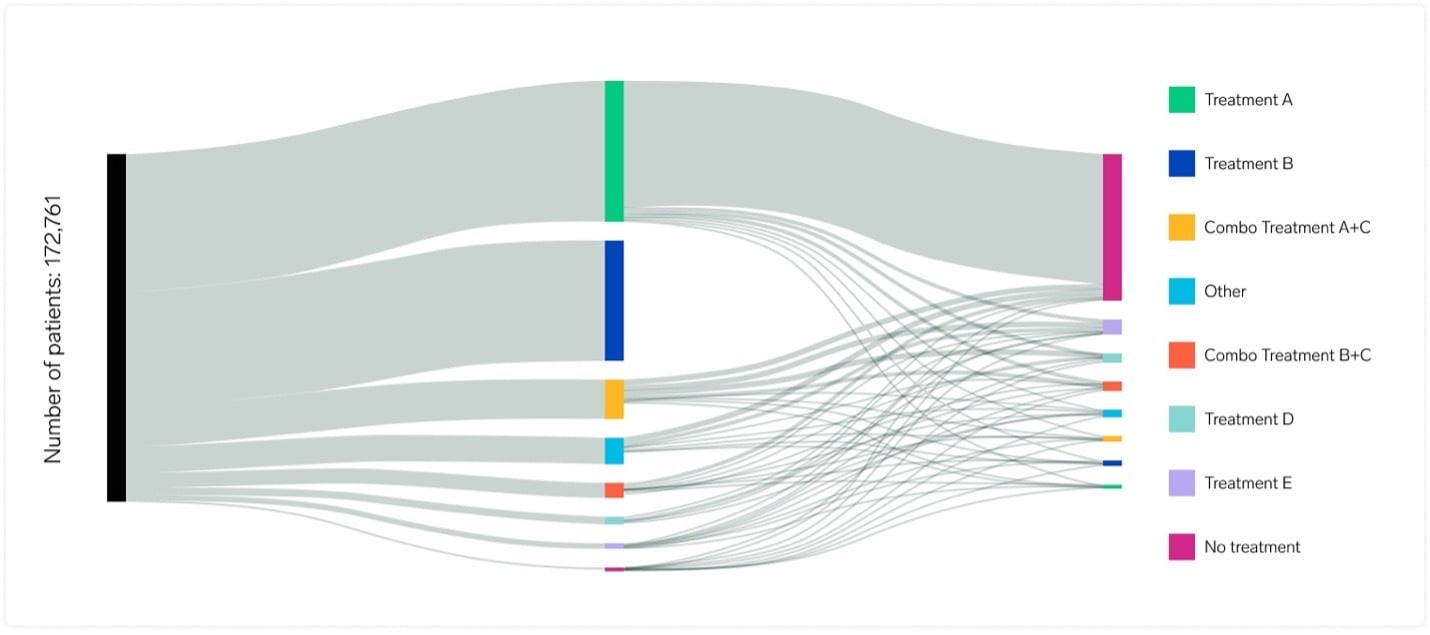
Real-world evidence, expertly delivered
"Aetion is well positioned to make a significant contribution to the studies and projects that we are leading, and as such, will help us raise awareness amongst regulatory agencies, HTA bodies, and payers about the increasingly important role real-world evidence is playing in healthcare decision-making.”
Managing Director, GetReal Institute
Read the Press Release
/20
Connect with us to work with the team advancing critical decisions in healthcare.