Discover
Visualize rapid, validated insights through real-world data.
Datavant Completes Acquisition of Aetion. Learn more →

Real-world evidence (RWE) is transforming how MedTech companies demonstrate product value, improve patient outcomes, and accelerate innovation. Through our platform and scientific services, we partner with you to:
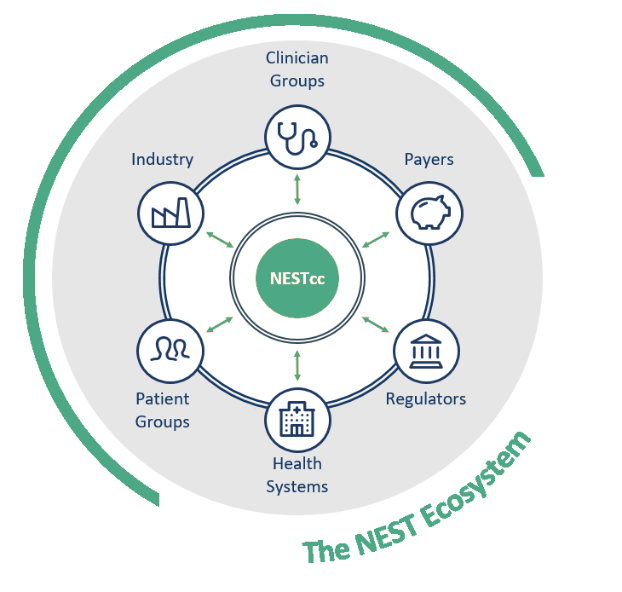
Through their strategic partnership, the National Evaluation System for health Technology Coordinating Center (NESTcc) and Aetion, along with the FDA’s Center for Devices and Radiological Health (CDRH), are on a mission to advance the use of RWE in the medical device and diagnostic ecosystem.

“This company is a $10B top line global medical devices company. Recognizing the importance of generating real world evidence (RWE) to support the clinical, economic and societal value of this company's portfolio and serve more patients, this company partnered with Aetion to develop a RWE Playbook to standardize and accelerate RWE generation. In the first phase of this transformative initiative, Aetion - in partnership with this company's core team - successfully organized and ran a first-of-its-kind workshop with global clinical and medical affairs leaders. The Aetion team delivered best in class thought leadership and content in how RWE studies should be designed across the product life cycle and drove great engagement amongst the participants. Aetion was able to balance the complex “theoretical principles” behind RWE, which can be overwhelming and complex, with this company's diverse product portfolio and evidence generation needs.”
Aetion offers generous benefits to its full-time employees, which currently can include:
The benefits offering may differ based on geographies where employees are based, when outside of the United States.
Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim veniam.