Academe, U.S. Food and Drug Administration (FDA) staff, and industry leaders gathered at the American Academy of Arts and Sciences on April 2 to discuss regulatory science challenges and advances, an annual meeting hosted by the Harvard-MIT Center for Regulatory Science.
The conversation ranged from milestones that contributed to drug regulation, e.g., the NEJM paper on Vioxx and the Nature paper on India’s clinical-trials system, to adaptive approaches and tools to bring drugs to market (see agenda).
Major themes from the meeting were:
- Regulatory science must take an agile approach;
- Drug repurposing can support public health; and
- There are methods and tools to increase confidence in RWE.
Regulatory science must take an agile approach
Like other fields adopting an agile approach to innovation, regulatory science relies on a shared vision to foster greater coordination and efficiency in drug development and commercialization—a principle reiterated throughout the day.
Gigi Hirsch, M.D., executive director of MIT’s Center for Biomedical Innovation, led a panel titled “Towards an ‘Agile’ Regulatory Framework.” Sanofi’s John Ferguson, M.D., spoke about the NEW Drug Development ParadIGmS (NEWDIGS) program, which uses demonstration projects to test development models and regulatory frameworks. Dr. Ferguson reported that NEWDIGS’ approach allows its members who have different incentives to coordinate collective thought processes and revise and regulate unambiguous terms. NEWDIGS’ flagship project inspired the European Medicines Agency’s adaptive pathways approach. Bluebird Bio’s Anne-Virginie Eggimann, M.Sc., shared she used it for early and frequent stakeholder involvement in a safe harbor environment on what information was needed in a clinical trial.
Drug repurposing can support public health
“It’s in the realm of public health to see if we can effectively repurpose drugs,” said FDA’s Joohee Sul, M.D., as doing so accelerates drug development, lowers costs, and improves outcomes for new patient populations, by using drugs and clinical data that already exist. And doing so in many cases will rely on real-world evidence, which FDA defines as ”the clinical evidence about the usage and potential benefits of risks of a medical product derived from analysis of real-world data.”
There are methods and tools to increase confidence in RWE
As FDA implements its plan to develop guidance on RWE, FDA clearly states it will continue to follow a shared learning process involving multiple stakeholders. Brigham and Women’s Hospital’s Sebastian Schneeweiss, M.D., Sc.D., chaired a panel on RWE and regulatory decision-making that covered a range of ongoing projects that represent shared learning. Scheeweiss was joined by FDA’s Jacqueline Corrigan-Curay, J.D., M.D.; Aetion’s Jeremy Rassen, Sc.D.; and Brigham and Women’s Hospital’s Jessica Franklin, Ph.D.
Dr. Corrigan-Curay discussed a range of demonstration projects on real-world data, RWE tools, and RWE study design, e.g., Limit JIA, IBD Plexus(R), and then summarized unmet needs regarding RWE and regulatory decision-making, saying next steps require:
- Capturing data directly from patients (the intent of FDA’s MyStudies Application);
- Knowing what transparency will look like for RWE just as we know what it looks like for RCTs;
- More discussion on the availability of covariates and on using covariates to balance risk;
- Empirical evidence to build confidence in RWE and enable guidance writing;
- Integrating standards into EHRs (the intent of FDA’s collaboration with UCSF’s Dr. Laura Esserman);
- More creative thinking and an open invitation for “all expertise.”
Drs. Rassen and Franklin also discussed best practices and demonstration projects:
- Dr. Rassen spoke on increasing confidence in RWE through good study practice and maximized transparency. For example, he shared a reporting template with design visualization to increase clarity of methods used in RWE studies and reduce review time for health care database studies (download the template here).
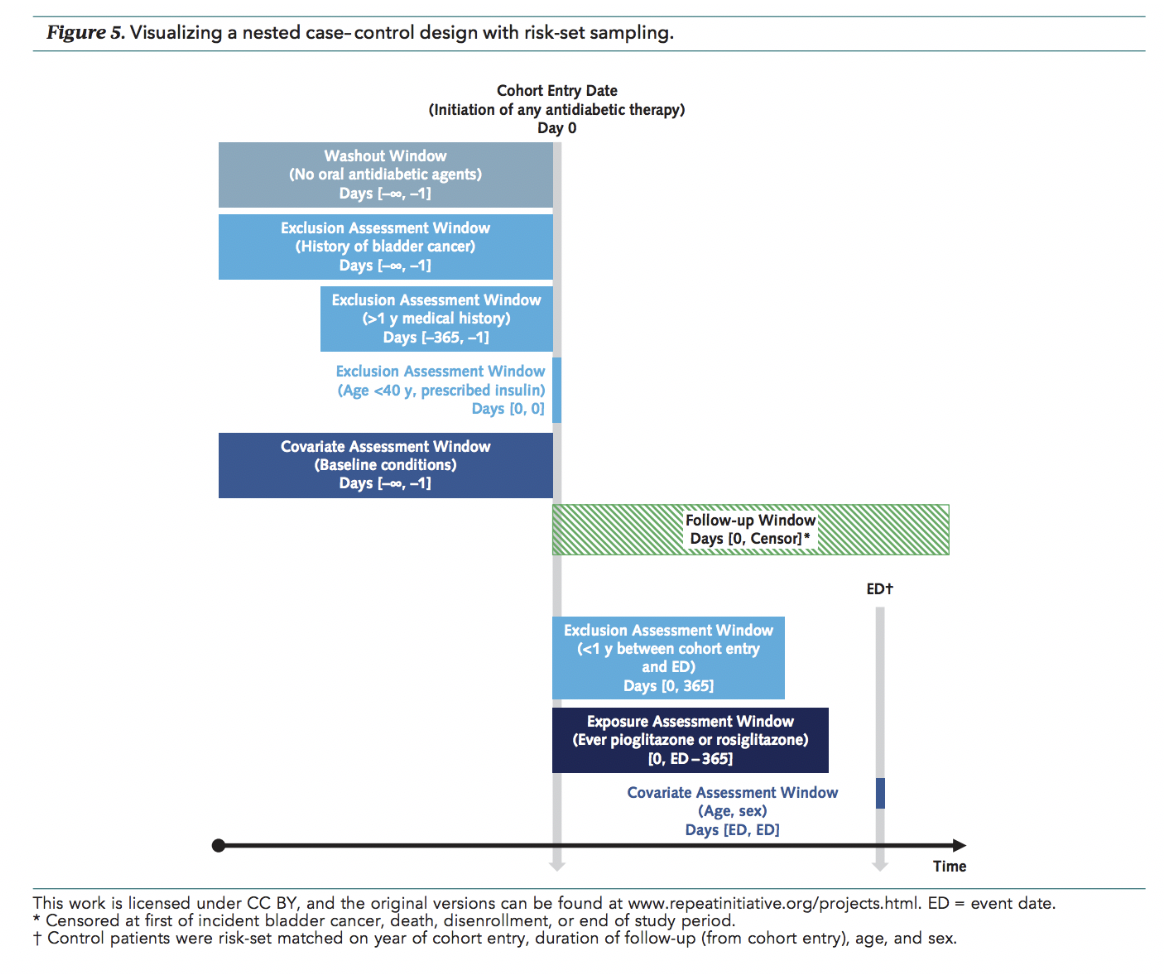
- Dr. Franklin spoke on anticipated learnings from RCT DUPLICATE, an FDA demonstration project which is using RWE to predict the results of seven ongoing Phase IV trials. This is the first time researchers will estimate the results of RCTs that have not yet concluded. Dr. Franklin anticipates learning about the characteristics of clinical questions that can be answered, the designs and analytic methods that provide the most robust estimates, and whether the implementation process supports the reliability, transparency, and reproducibility of RWE.
The day exemplified the shared learning process that will ultimately produce FDA’s guidance on RWE due in 2021.

